Story of Lipid Movement
Lipids are one of the major sources of energy and have many other important functions like synthesis of membranes and signaling molecules. For all these purposes fatty acids need to be released from their esterified forms. Triglycerides are a major storage form of fatty acids, and are packaged with lipid droplets. The degradation of lipid droplets to fatty acids was mainly thought to involve cytosolic lipases and autophagy, depending on the cell type. Recently, a direct lipid droplet to lysosome transfer of lipids has been described although the players involved in this process remain unknown. In this article, the researchers describe that a small GTPase, ARL8B, plays an important role in this phenomenon.
ARL8B, has been very well characterized for its role in lysosome positioning and fusion with other vesicles. ARL8B was also previously reported to be increased on lipid droplets during Mycobacterium tuberculosis infection but its role on lipid droplets was not known.
In this article, the researchers show how the switch from GTP to GDP changes the binding of ARL8B with lysosomes to lipid droplets, and interaction between these two acts as a point of contact for the two. As a result, the release of fatty acids can directly happen inside the lysosomes instead of inside the cytoplasm. It is interesting that this process directly impacts the abundance and size of lipid droplets and at the molecular level, impacts specifically the poly-unsaturated fatty acid containing triglycerides. The significance of this process needs to be further evaluated as it may have implications in infections, atherosclerosis, or fatty liver disease- conditions which involve the accumulation of triglycerides.
ARL8B mediates lipid droplet contact and delivery to lysosomes for lipid remobilization
One RNA binding protein, splicing, and fate of a pluripotent stem cell
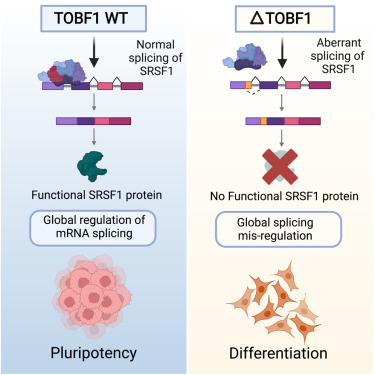

Embryonic stem cells have the capacity to divide indefinitely and to form all the body cells via differentiation. There have been numerous studies elucidating the factors that are responsible for maintaining the stemness of the pluripotent stem cells, i.e pluripotent stem cells giving rise to another pluripotent stem cell instead of a differentiated cell. Long non-coding RNAs have found a place in these studies and there have also been reports of RNA binding proteins playing a crucial role in maintaining the stemness. The current study looked at one such RNA binding protein, TOBF1, to better understand the process.
Many of the lncRNAs have been shown to affect the transcription and post transcriptional events but alternative splicing is one that has not been described in much detail. In this study, it was found that TOBF1 associates with one of the major splicing factors and thus regulates the formation of various isoforms of proteins that help in maintaining the stemness.
The Paradox of Melanin Synthesis
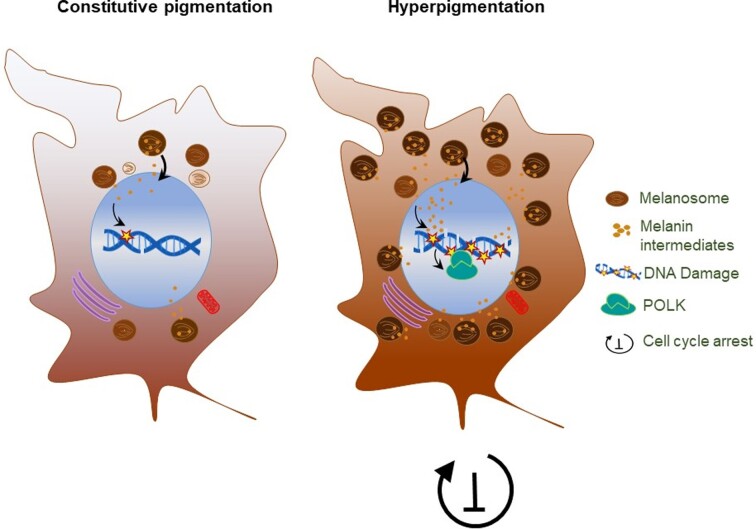

Each of us has experienced tanning as a result of prolonged sun exposure. Melanin synthesis, responsible for this tanning effect, acts as a shield safeguarding the skin from DNA damage induced by UV light. Surprisingly, it has been observed that this protective process for the skin can actually harm the cells responsible for producing melanin—melanocytes. The conventional perception of melanin synthesis as a skin protector conceals its adverse effects on melanocytes.
Several by-products generated during melanin synthesis trigger oxidative DNA damage. UV-induced DNA damage causes an accumulation of mutations in genomic DNA. This study sheds light on the role of a non-canonical DNA polymerase, Pol κ, which becomes active during prolonged pigmentation. Pol κ activates the replication stress response, preventing uncontrolled cell proliferation in the presence of damaged DNA. The absence of Pol κ in melanocytes results in genomic instability, potentially leading to excessive melanocyte proliferation and the development of melanoma. This study highlights an unknown link between melanin and melanoma, emphasizing the protective role of Pol κ in melanocytes. – Jeyashri Rengaraju
Hcy-induced neural damage rescue
Homocysteine (Hcy) is a molecule produced in our cells as part of the normal breakdown of certain amino acids. When there is an excessive buildup of Hcy in the body, a condition known as hyperhomocysteinemia (HHcy) develops. It can lead to damage in blood vessels, brain degeneration, and cognitive decline. In this research, a model of HHcy in nerve cells, as well as in zebrafish, was used to show that high Hcy levels impairs autophagy. This leads to nerve cell damage characterized by problems in the function and structure of mitochondria and even cell death. The researchers found that this mitochondrial dysfunction is triggered by the accumulation of damaged proteins due to Hcy-induced stress in the endoplasmic reticulum (ER). We discovered that this stress is sustained because it disrupts the normal autophagic process and is further aggravated by the activation of a protein called mTOR. By alleviating ER stress, they were able to rescue the cell damage caused by Hcy. Similarly, by boosting autophagy, they could reduce the buildup of damaged proteins and ER stress, ultimately protecting nerve cells. The study suggests potential strategies for treating neural and cognitive problems associated with HHcy by either promoting autophagy or preventing ER stress.
VitB12 deficiency and cardiovascular disease risk
The health and well-being of offspring are influenced by a multitude of factors, including genetic, environmental conditions, and parental care. Among these factors, parental nutritional status has emerged as a critical determinant of offspring health. The quality and quantity of nutrients available to parents before and during conception, as well as throughout pregnancy and lactation, can significantly impact the long-term health outcomes of their offspring. Thus, balanced nutrition, attained through a proportionate intake of macro and micronutrients in the diet of parents is essential for offspring health. Vitamin B12 (B12) is one such essential micronutrient that plays a fundamental role in DNA synthesis and metabolism.
Maternal vitamin B12 deficiency plays a vital role in fetal development. Recognizing that B12 insufficiency in human populations is typically subclinical in severity, in this current study, the effects of diet-induced maternal vitamin B12 deficiency on F1 offspring in terms of cardiometabolic health and normalization of these effects by maternal-periconceptional vitamin B12 supplementation were assessed.
In this study, when Vit B12 deficient females were mated with males that had normal B12 levels produced offspring that showed reduced B12 levels.
The most interesting finding of the study was that although there was a similar B12 depletion in male and female offspring, adverse cardiometabolic risk factors were only elevated in male offspring.
Thus maternal vitamin B12 deficiency has a programming effect on the next generation and increases the risk for cardiometabolic syndrome in a sex-specific manner. – Praveen Singh

